- Surprised, Flash Point correction of 8.5 Deg C at Leh, Jammu & Kashmir due to high Altitude
- Surprised, Flash Point correction of 8.5 Deg C at Leh, Jammu & Kashmir due to high Altitude
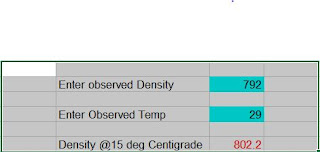
Absolute Flash Point of a Kerosene Sample was observed 29 deg C at Leh (altitude 3,256 m (10,682 ft) above mean sea level.) for product dispatched having Flash Point 38 deg C at Ambala. Later on the issue was studied and concluded that air pressure correction at Leh is about 8 deg C. Known boiling point, Flash Point etc reduce as altitude increases, such high correction was first much beyond expectation.
Standard Fortin's Barometer design ( Barometer Specifications) is inadequate since altitude is >3000 meters at Leh. Subsquently air pressure was digitally measured and concluded that air pressure of 67.8 kPa gives correction of 8.5 deg C in flash point as per Petroleum products standard test method P:20 and IP 170 etc.. The correction equation given in these test methods including ASTM 93, IP 34 restricts use of equations between 98 kPa & 104.7 kPa. At sea level, air pressure is 101.3 kPa ( 760 mm, 1013 mbar etc).
Boiling point of water was observed 88.5 deg C at Leh compared to 100 deg C at sea level and standard conditions.
May find the detail studied data interesting in next post.
Author's Profile
List of all blog articles on Petroleum QC by RJ Patel.
Author's Profile
List of all blog articles on Petroleum QC by RJ Patel.
Comments
Please share some knowledge with Change in RVP in Gasoline with increasing percentage of Ethanol.
RVP of Ethanol is less than Gasoline, but it increases RVP of Gasohol( 10 % Ethanol Blended in Gasoline). Please help me to understand the possible reaction/ reason behind.
regards,
Priyanka Agarwalla
Thanks for your comments and let me say sorry for lack of my understanding of how the comments appear in blog. Today only I could see your comment. Regarding your query, let me assure you, very soon I will post one exclusive issue on your stated interest o RVP due to ethanol. Thanks once again and please bear it for some time.
despite the fact that fuel grade ethanol has a lower vapor pressure than gasoline (see Figure 1).
The low vapor pressure of fuel grade ethanol is caused by attractive forces between the ethanol
molecules. The strongly electronegative oxygen atom in each ethanol molecule is attracted to
the somewhat positive hydrogen atoms in other ethanol molecules. The attraction between
ethanol molecules means that it has a stronger tendency to stay as a liquid and not vaporize into
the more dispersed gaseous state. However, when blended into gasoline at relatively low
concentrations the more numerous gasoline molecules disrupt the attractive forces between
ethanol molecules and allow the ethanol to readily evaporate, raising the vapor pressure of the
blend. Not surprisingly this increase in vapor pressure with ethanol is more marked with the
lower RVP hydrocarbon blendstocks. This would be true with the addition of any component
which raises vapor pressure, as the final pressure is a weighted average of the pressure
contributions of all of the components. As ethanol content is increased above about 20% the
vapor pressure increase becomes less, and above about 50% ethanol vapor pressure for the blend
is less than that of the gasoline.
Ravi